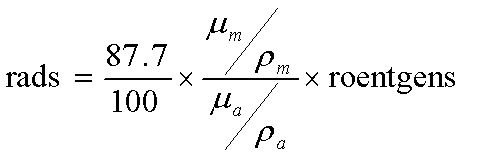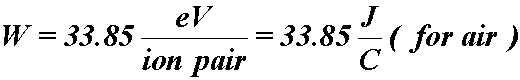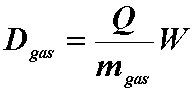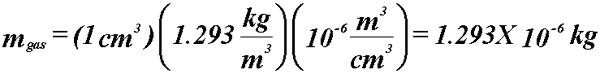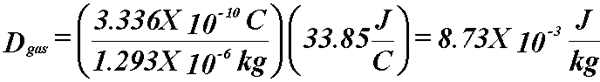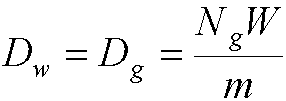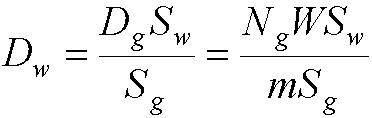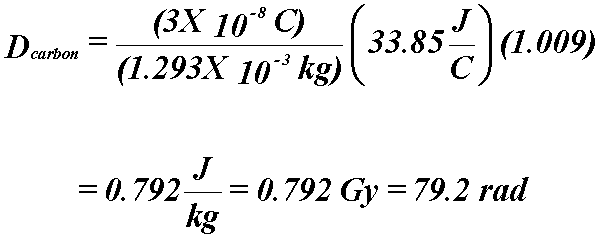Topic 5 - Dose Units
Radiation Dosimetry |
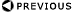 |
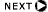 |
Dosimetry Overview
- Units
- Specific Gamma-Ray Emission
- Beta Radiation
- Internally Deposited Radionuclides
- Neutrons
Introduction
- Dosimetry attempts to quantitatively relate specific radiation measurements
to chemical/biological changes that could be produced
- Essential for quantifying biological changes as a function of radiation
received
- For comparing experiments
- For other purposes
- Radiation interaction
- Produces ionized and excited atoms and molecules
- Secondary electrons
- Produce additional ionizations and excitations
- Finally all energies are expended.
- Initial electronic transitions rapid (<10-15s)
- Represent the initial physical perturbations from which all effects
evolve.
- So, ionization and energy absorption are the starting point for radiation
dosimetry
Quantities and Units
- Absorbed Dose
- Primary physical standard in dosimetry
- Defined as energy absorbed per unit mass from any kind of ionizing
radiation in any target.
- SI Unit of absorbed dose,
- Called the gray (Gy)
- 1 Gy = 1 J kg-1
- Historical (older) term
- 1 Gy = 107 erg/103g = 104 erg/g
= 100 rad
- Exposure
- Defined for X and gamma radiation
- In terms of ionization of air
- Old unit called “roentgen” (R)
- Initially defined in 1928, current definition is:
- 1 R º 2.58 x 10-4C kg-1 of air, exactly
- Applies only to electromagnetic radiation; the charge and mass
refer only to air.
Roentgen - original definition
- Amount of radiation that produced 1 esu of charge in 1 cm3
of air at STP
- 1 esu = 3.335 x 10-10C

- At STP air has a density of 0.001293 g cm-3
- 1 kg of air has a volume of 7.734 x 105 cm3
- 1 R = 3.335 x 10-10 C cm-3 of air
- How much energy is absorbed in air from 1R?
Absorbed Dose and Exposure
- What is the absorbed dose in air when the exposure is 1 R?
- Need to know W for air
- 1 R º 2.58 x 10-4C/kg x 33.7 J/C = 8.8 x 10-3
J kg-1
- This equals 8.8 x 10-3 Gy (0.88 rad)
- Similar calculations show that 1R would produce a dose of
9.5 x10-3 Gy (=0.95 rad) in soft tissue.
- Why is there a difference between air and tissue???
- This is why one can say that 1 R ~ 1 rad in tissue.
Exposure Measurement - Free Air Chamber
- Feasible to measure exposure at radiation energies between few keV
and several MeV
- Definitive measure is by laboratory device known as free air chamber
- X-ray beam enters through a portal and interacts with cylindrical
column of air defined by entry diaphragm
- Ions created in defined space are measured
Parallel Plate Free-air Ionization Chambers
Free Air Chamber
- Photons enter chamber and interact with fixed quantity of air
- Ions from air collected by plates
- Lead lined (shielded)
- Electric fields are kept perpendicular to plates by guard rings and
guard wires
- Guard wires assure uniform potential drop across plates
- Field intensity is ~ 100 V/cm
- Collect ions prior to recombination
- Voltage low enough so no secondary ionizations
- Current flow is measured
- All energy of primary electrons must be deposited in sensitive volume
of air for meter to work properly
- What if all primary electrons are not collected in sensitive volume?
- If equal number coming in from outside of sensitive volume as is
going out: Electronic Equilibrium
Electronic Equilibrium
- For every electron which escapes the sensitive volume, another electron
of equal energy enters the sensitive volume and deposits energy in the
detector
- A layer of air between entrance port of free air chamber and the
sensitive volume can provide enough air so that electronic equilibrium
is attained
Free-Air Ionization Chamber
Wide-Angle Free Air Chamber (WAFAC)
- NIST primary standard for low-energy photon-emitting brachytherapy
sources such as 125I.
- Variable volume, circular free-air chamber,
- Symmetrical about the beam axis, with lines of force parallel
to the beam axis.
- 80 mm diam. Tungsten aperture (not visible at right) chamber
used to define the beam.
- 3 electrodes: polarizing, middle, and collecting electrode
(farthest from the source and left in the picture) about 150 mm
in diameter.
- Chamber volume varied from ~75 cm3 to 804 cm3
by changing middle electrode from 11 mm to 152 mm.
Measuring Exposure
- Free air chamber practical only as laboratory device
- Portable instrument needed
Exposure Measurement: Air Wall Chamber

- Practical alternative to free-air chamber
- Built as a capacitor
- Central anode, insulated from rest of chamber
- Given an initial charge
- When exposed to photons, 20 electrons neutralize charge
& lower potential between anode and wall
- Change in potential difference is proportional to total ionization
(and therefore exposure)
- Better for field use than the Free Air Chamber
- Simulates compressing air into a small volume by using ‘air equivalent’
material
- X-ray absorption properties similar to that of air
- Walls must be thick enough to generate enough primary electrons
- Walls must be thin enough so that primary radiation is not shielded
- Ideal air wall chambers have only primary electrons ionizing the
air in the sensitive volume
- Ideal wall thickness is almost energy independent over a range from
200 keV to almost 2 MeV (See figure 6.4)
- Greater than 3 MeV primary electrons have long range
- Impractical to build air wall chamber of sufficient size
- When walls are made thick enough to generate primary electrons,
radiation is attenuated significantly
- Radiation intensity will no longer be constant
- Primary electrons not produced uniformly
- No electronic equilibrium
Exposure-Dose Relationship
- Exposure
- measures charge produced in a mass of air
- C/kg
- Absorbed dose
- Measures energy absorbed per mass
- J/kg
- How to relate measurement in air to absorbed dose in something besides
air?
- Energy absorption in air ¹ energy absorption in tissue
- Dose in air ¹ dose in tissue
- 1 R = 87.7 ergs/gair = 95 ergs/gtissue
- 1 rad = 100 ergs/gtissue
- For regulatory purposes, frequently 1 R is assumed to be equal to
1 rad
- Conversion can be done if required
Exposure to Dose Conversion
- Equation 6.12
- mm = Energy absorption coefficient for tissue
- ma = Energy absorption coefficient for air
- rm = Tissue density
- ra = Air density
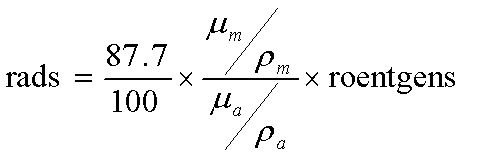
Bragg-Grey Theory
- How to measure absorbed dose?
- The best way would be calorimetry...but not very practical. Instead,
absorbed dose is measured by:
- measuring ionization
- use of correction factors
- calculating (approximating) dose
- This is done with BRAGG-GREY CAVITY THEORY
Measurement of Absorbed Dose
- Bragg-Gray principle relates ionization measurements in a gas to
absorbed dose in some material.
- Consider a gas in a walled enclosure irradiated by photons:
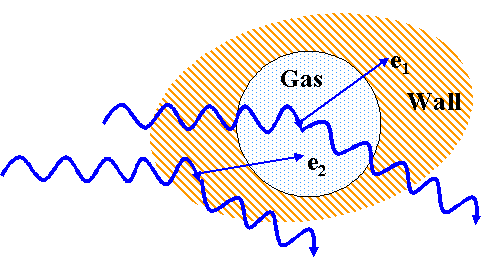
- Photons interact in cavity and wall
- Chose wall material that has similar radiation absorption properties
as tissue (e.g., Z)
- Cavity is very small
- (doesn’t change angular and velocity distributions of 20
electrons)
- “Electronic equilibrium” exists in cavity
- (# e- stopping = # e- starting in cavity)
- requires wall thickness > range of 2o e
- Ionizations in the gas
- Can measure the charge liberated.
- If you know the energy required to ionize the gas,
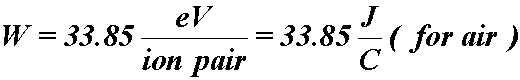
- Then the dose to the gas is:
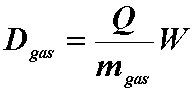
- where,
- Q = coulombs of charge liberated
- W = average ionization energy for the gas
- m = kg of gas in the cavity
- Example: A cavity filled with (1 cm3) air at STP is exposed
to a radiation field that liberates 3.336X10-10 C. What
is the dose to the air?
- At STP:
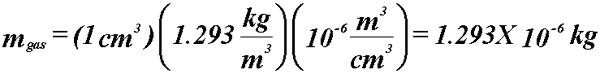
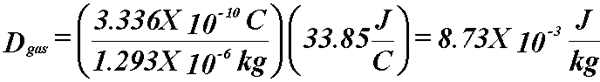
- Know the dose to the gas.
- What about the dose to the medium surrounding it?
- Assume our cavity is really small...small enough that it does
not disrupt the electron spectrum.
- Wall thickness must be as great as range as secondary charged particles
(not too great to attenuate beam)
- Then energy absorbed per unit mass of wall is related to that absorbed
per unit mass of gas by:
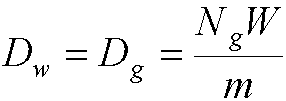
- Note - this special case is where the wall and gas are the
same type of material
- Dw is the dose to the wall
- Dg is the dose to the gas
- Nq is the number of ions produced in the gas
- W is eV required to produce and ion pair
- m is the mass of gas in the cavity
- If gas and wall don’t have same atomic composition, a slight modification
is required:
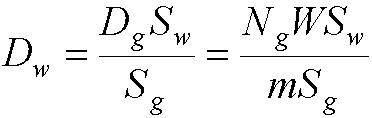
- where Sg,w are the mass stopping powers of the wall and
the gas
- the cavity and gas pressure must be small
Example
- 1 cm3 of air in a block of carbon is exposed to 60Co
γ
- What is the absorbed dose to the carbon?

- Mean mass stopping power ratio for 60Co γ in carbon
relative to air (Table 6.1) = 1.009
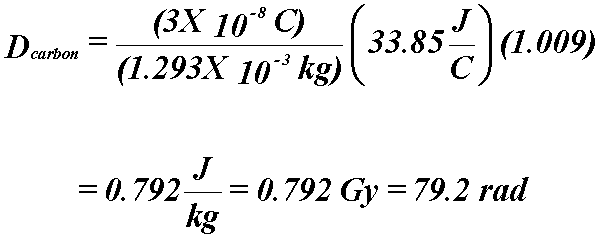
- This equation allows us to measure the ionizations in a gas and relate
it to dose to the medium.
- If neutrons are present, the wall must be at least as thick as the
maximum energy range of any secondary charged recoil nuclei produced
by the nuclear interactions.
- Chambers that meet these conditions can be used to measure absorbed
dose to the medium
|