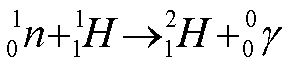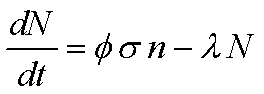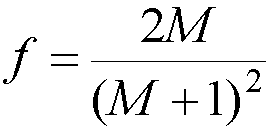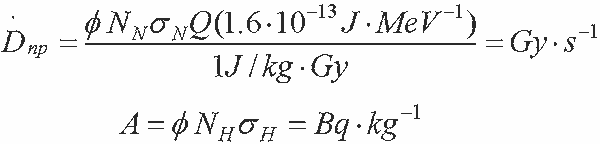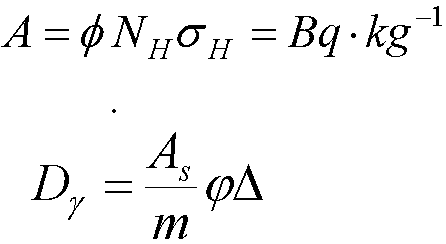Topic 5 - Dose Units
Neutrons |
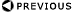 |
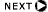 |
Neutron Production
- Nuclear Reactors
- Fission neutrons from U or Pu
- Accelerators
- Combined sources
- Radium & Be
- Polonium & Be
Neutron Classification
- Thermal
- In thermal equilibrium with their environment
- Distributed according to Maxwell Boltzman distribution
- Most probable energy ~ 0.025 eV at 20 C
- Higher energies: 0.01 or 0.1 MeV
- Slow, or intermediate or resonance
- Fast neutrons have energies up to ~10-20 MeV
- Relativistic neutrons have still higher energies
Interaction with Matter
- Uncharged like photons
- Travel appreciable distances without interacting
- Attenuated exponentially under good geometry
- Don’t interact appreciably with electrons
- Collide with atomic nuclei in elastic and inelastic collisions
- Elastic collisions:
- Total energy is conserved
- Energy lost by neutron is equal to total energy of recoil nucleus
- Inelastic collisions
- Nucleus absorbs some of the energy and is left in an excited
state
- Fast neutrons
- Series of mostly elastic scattering reactions
- Slowing down process is called moderation
- As energy decreases, scattering continues but probability of
capture by another nucleus increases.
- If neutron reaches thermal energies it will randomly move around
until absorbed by a nucleus.
Important Neutron Reactions
- 1H(n,g
)2H
- Example of radiative capture
- Neutron absorption followed by immediate emission of gamma photon.
- Explicitly:
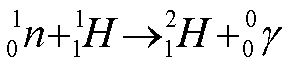
- The photon has an energy of 2.22 MeV
- Of significance when tissue exposed to thermal neutrons
- 14N(n,p)14C
- Cross section is 1.78 barns for thermal neutrons
- Q value is 0.626 MeV
- Significant contributor to dose when tissue is irradiated by
neutrons
- Since p and recoil nucleus have limited range, energy deposited
locally.
- Neutron Activation
- Production of radioactive isotope by absorption of neutron.
- Equation dictating creation of radioactive isotope:
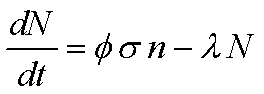
- Where
- f is the flux, neutrons per cm2 per
s
- s is the activation cross section,
cm2
- l is the decay constant of the produced isotope
(s-1)
- N is the number of produced atoms
- n is the number of target atoms.
Absorbed Dose from Neutrons
- Concerned primarily with
- Neutron interaction with tissue elements H, C, O, N
- Resultant dose
- Neutrons interactions
- produce HCPs
- short range
- CPE occurs
- Therefore, dose = kerma (for En <= 20 MeV)
Fast Neutrons

- f(E) flux, n cm-2s-1
- E, neutron energy in Joules
- Ni, atoms per kg of the ith element
- sI, scattering cross section, barns*10-24 cm2
- f = fractional energy transferred from neutrons
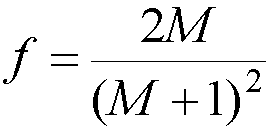
- Isotropic scattering
- Average fraction of neutron energy transferred in elastic collision
- Nucleus of atomic mass number M
Thermal Neutrons
- Two reactions:
- 14N(n,p)14C
- 1H(n,g)2H – uniformly distributed g isotope
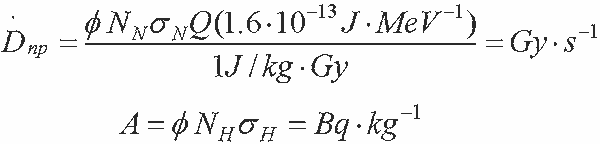
Thermal Neutrons
- 1H(n,g)2H dose rate
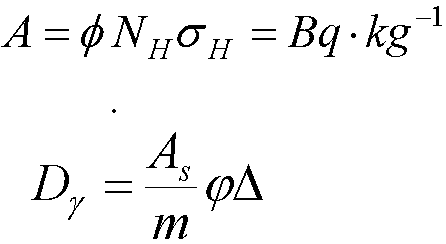
- Absorbed fraction, j
- Emission fraction (energy, abundance, conversion factor), D
Neutrons Dose Summary
- Divide into two types of dose calculations
- Fast neutrons with multiple energy groups
- Thermal or slow neutrons
|