Topic 4 - Sources of Radation
Radioactivity & Natural Radiation Sources |
 |
 |
Radioactivity
- Spontaneous decay (disintegration) of the NUCLEUS of
an atom
- Involves energy changes in the NUCLEUS
- Results in:

Band of Stability
- The Band of Stability
There is no known formula to predict if an isotope will be stable.
- Example 56Fe has highest binding energy per nucleon
- 53Fehas a half-life of tĹ = 8.5 minutes
- 60FetĹ = 300,000 years
Nuclear Stability Curve
- Rule of thumb for predicting stability
- Plot all known isotopes (tĹ > 10-8 s)
as no vs p+
- two lines can be drawn to enclose all of the stable nuclei
- Between these 2 lines lies the Band of Stability

- no/p+ ratio increases as atomic number increases.
- As more protons packed into nucleus, larger numbers of neutrons required
to compensate strong force - to "dilute" the proton-proton
electrostatic repulsions.
- Isotopes above and to the left tend to Ŗ -emitters.
- Isotopes below and to the right tend to be positron emitters.
- Isotopes above atomic #83 tend to be a emitters.
Stability Quirks
Odd Even Rule
- If nís and pís are both even, isotope is likely stable†
- Of 264 known stable isotopes
- only 5 have both odd numbers
- 157 have both even numbers
- 102 have both an odd and an even number.
- The odd-even rule is related to the spins on the nucleons:
- Both p+ and no have spins. When two like
particles have paired spins, combined energy is less than when
their spins are not paired.
- When even numbers of p+ and no all the
spins can be paired and the system has less energy (i.e. more
stable) then when an odd proton or odd neutron is present.
Magic Numbers
- Isotopes with specific numbers of protons and neutrons are more
stable then the rest.
- Numbers are 2, 8, 20, 28 , 50, 82, and 126.
- When both the numbers of the p+ and the number of
no are the same magic number the isotope can be quite
stable.
- eg. 42He, 168O,
4020Ca
- All these isotopes are extremely stable.
- †20882Pb has 82 p+ and 126
no
- Existence of magic numbers supports the hypothesis that there are
nuclear energy levels similar to electron energy levels.
The facts of life
- Where do radioactive (and other) elements come from?
- Universe is primarily hydrogen and helium
- Big Bang produced mainly H and He, with trace amounts of some
lighter elements
- No appreciable production of C, N, O, Fe, Mg, Si, ... , and other
elements heavier than iron.
- Where did these elements come from?
- Most of the elements lighter than iron and nickel can be built
up from successive rounds of thermonuclear fusion burning in the
cores of stars.
Cosmic Abundance
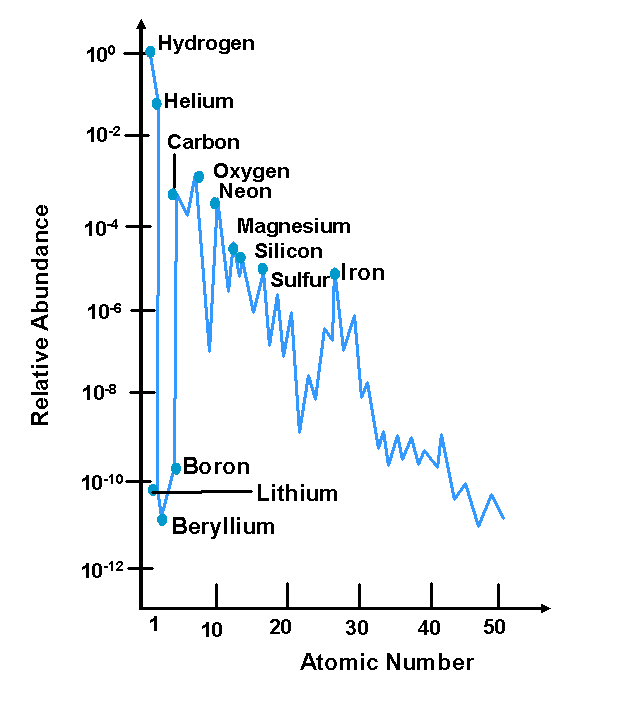
Elemental Distribution
- Extreme abundance of hydrogen
- General decrease with Z
- Low abundance of some elements (Li, Be, B, Sc)
- High abundance of Fe, Ni, Pb
- Greater abundance of even Z vs odd Z
- The process of creating the heavier elements is called nucleosynthesis
. Itís from neutron capture during a supernova
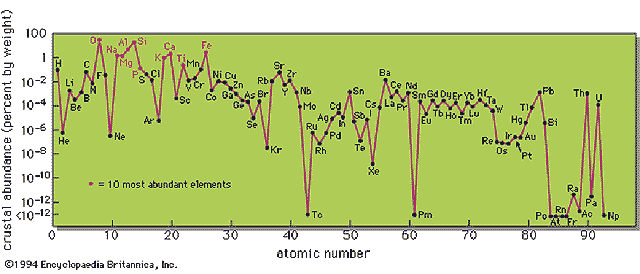
Crustal Abundance of Elements
Radiations/Radionuclides
- Solar/Cosmic Radiations
- Terrestrial radionuclides
- Primordial
- Chain decay
- Other
Natural Sources of Ionizing Radiation
- Cosmogenic radionuclides, 3h, 14C, 7be,
- Cosmic radiation, P+, e-, …
- Terrestrial radiation, 232Th, 238U, 226Ra, 40K, 87Rb
Cosmic Radiation
- High energy particles
- 87 % protons
- 1% Electrons
- 11% alpha
- Muons
- 1% Heavy particles (4 < Z < 26)
- Interact with earthís atmosphere
Cosmic rays
- Altitude dependent
- Latitude dependent
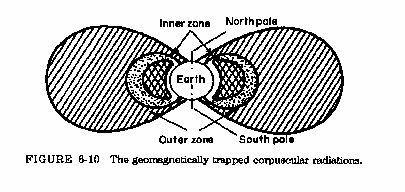
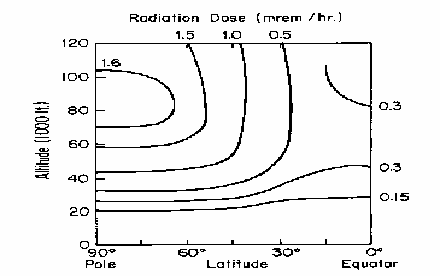
Solar Radiation
- High energy protons and electrons streaming from solar flares hit
the earthís outer atmosphere
Cosmogenic Nuclides
- Induced Radionuclides
- Produced by cosmic ray interactions with atmospheric nuclei
- Includes 3H, 14C, 7Be.
- Also† 10Be, 22 Na, 32P, 35S, 39Cl
| Nuclide |
Half-Life |
Source |
| 14C |
5730y |
14N(n,p)14C |
| 3H |
12.3y |
Cosmic ray interactions with N and O spallation from cosmic-rays,
6Li(n,alpha)3H |
| 7Be |
53.28 d |
Cosmic ray interactions with H and O |
Primordial Radionuclides
- Primordial radionuclides are left over from when the world and the
universe was created.
- They are typically long lived, with half-lives often on the order
of hundreds of millions of years.
Radiation From the Earth
- Naturally occurring radionuclides present in rocks, soils, plants,
water, air, and building material
- Major nuclides include
- Uranium (U)
- Thorium (Th)
- Radium (Ra)
- Radon
Primordial nuclides - examples
| Nuclide |
Half-Life |
Typical Activity |
| 235U |
1.04 * 108 yr |
0.72% of all natural uranium |
| 238U |
4.47 * 109 yr |
99.2745% of all natural uranium; 0.5 to 4.7 ppm total uranium in
the common rock types |
| 232Th |
1.41 * 1010 yr |
1.6 to 20 ppm in the common rock types with a crustal average of
10.7 ppm |
| 40K |
1.28 * 109 yr |
soil - 1-30 pCi/g (0.037-1.1 Bq/g) |
Thorium Series (4n)
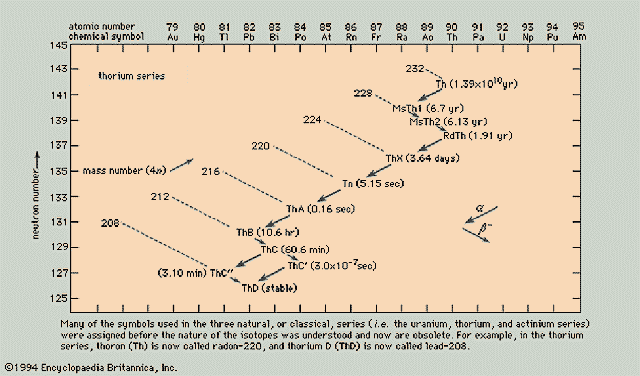
Neptunium Series (4n+1)
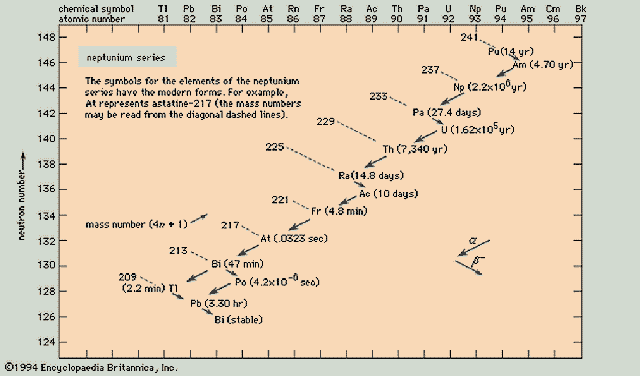
Uranium Series (4n+2)
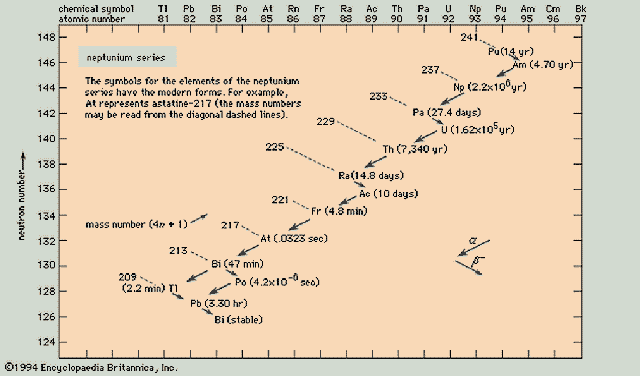
Actinium Series ( 4n+3)
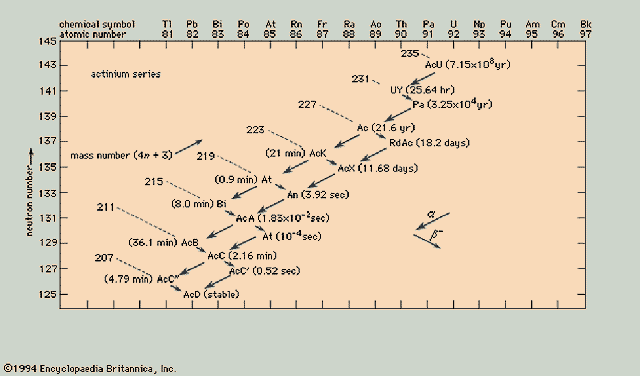
|










